
Our offers
echOpen Start
-
echOpen O1 Probe
€980 ex. VAT
- Annual commitment €300 ex. VAT
- Digital Features: Secure App & Cloud
- Digital training content
- 1 license included
- Livraison 30€ HT
-
Total
1 578€ TTC

echOpen Pro
-
echOpen O1 Probe
€980 ex. VAT
- All inclusive over 5 years €1 500 ex. VAT
- Digital Features: Secure app and cloud
- Remote or in-person training
- Continuous updates and new features
- Measurement tool (available in 2026)
- AI: Bladder (available in 2026)
- Premium customer support & 3 licenses included
- Livraison 35€ HT
-
Total
2 976€ TTC
An all-inclusive offer
- Get access to more than 30 detailed training sheets, at no extra cost.
- Read our in-depth, regularly updated training articles by certified ultrasound experts.
- Attend our monthly webinars and learn POCUS step-by-step with each use vase.
- Take part in our weekly live exchange sessions with our specialists.
- Customer service based in France.
- 12‑month warranty with replacement by a new unit.
- 24-month warranty covering manufacturing defects.
- 14‑day trial period with a full refund guarantee after receiving the probe.
- Quarterly updates included.
- Distance assessment tool coming early 2026.
- Progressive integration of AI: liquid volumes (Q4 2025) and LVEF (2026).
- Continuous improvements to image quality.
Our use cases
- Lungs: Assess for basal pleural effusions and visualize interstitial syndromes (B‑lines) to evaluate edema.
- Heart: Assess for pericardial effusions (subxiphoid or parasternal view).
- Limitations: Not suitable for the fine diagnosis of valvular heart diseases or for precise ejection fraction.
- Free fluid: Search for peritoneal effusions (ascites, hemoperitoneum) in the Morison's pouch, splenorenal space, and the Douglas pouch.
- Urinary system: Search for bladder distension (retention) and dilation of the pyelocaliceal cavities (hydronephrosis).
- Gallbladder: Detect stones or dilation of the gallbladder (diagnostic orientation).
- Abdominal Aorta: Screen and monitor the aortic diameter for the detection of aneurysms (AAA).
- Inferior Vena Cava: Visualize the IVC to rapidly estimate volume status and central venous pressure.
- TVP: Perform a compressibility test of the deep veins (femoral and popliteal) to assess patency.
- eFAST Protocol: standardized assessment for pericardial, pleural, and peritoneal effusions in trauma patients.
- Decision-making algorithms: Assistance with rapid triage in cases of dyspnea, chest pain, or undifferentiated shock.
- Vascular access: Anatomical landmarking (jugular, subclavian, femoral, basilic veins) to identify the side before puncture.
- Safe punctures: Localise fluid collections (ascites, pleural effusion) to identify the optimal puncture site and avoid at‑risk organs.
- Contraindication: This device is not intended for biopsy guidance or for use in complex operating room procedures.
echOpen gallery
Assess image quality.
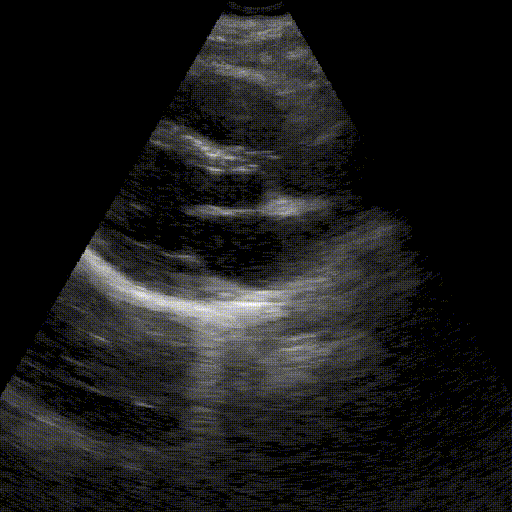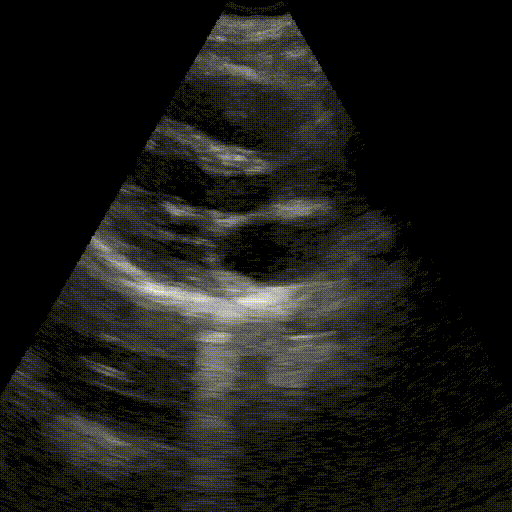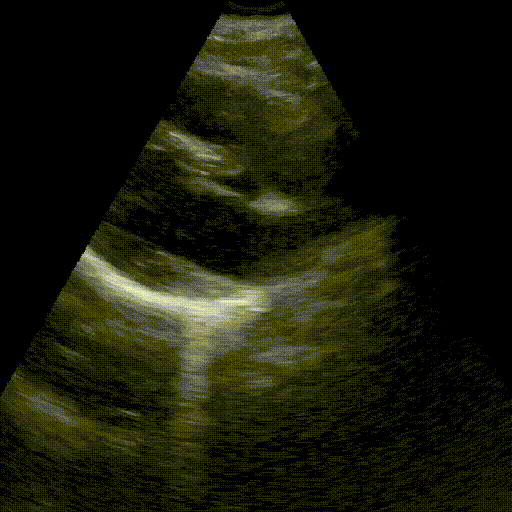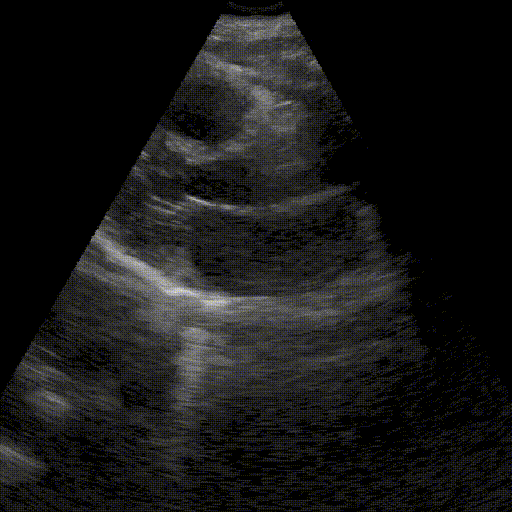
Heart

Liver
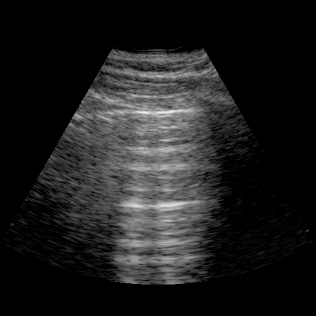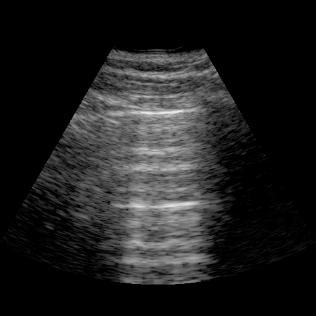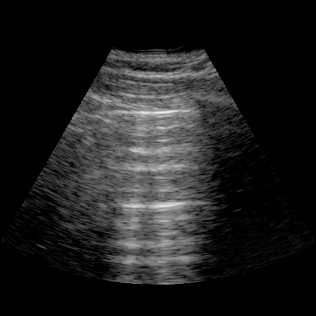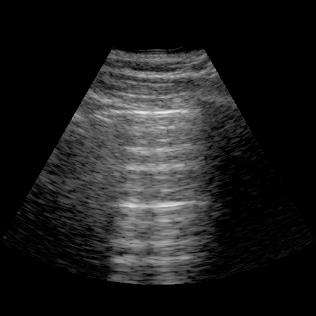
Lungs
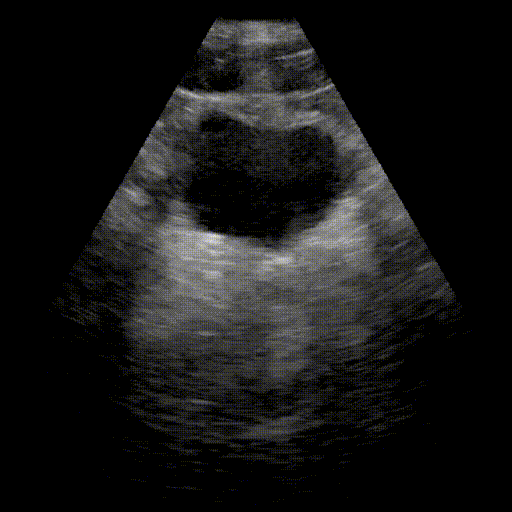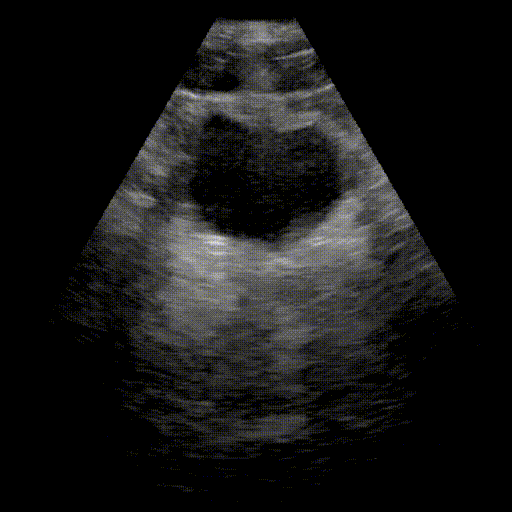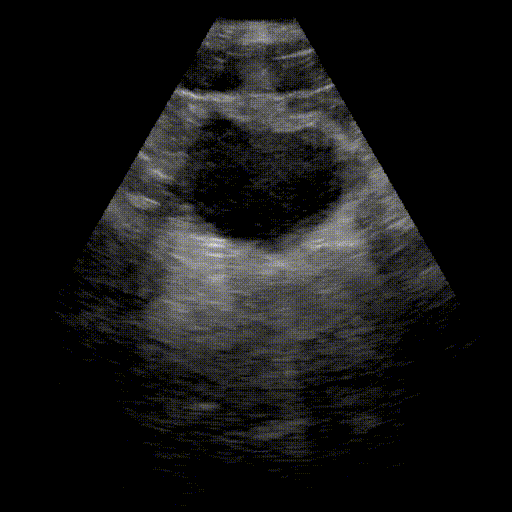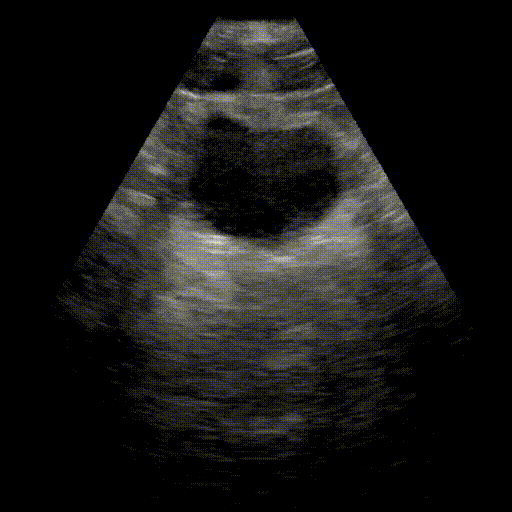
Bladder
Your frequently asked questions
The box contains your echOpen O1 tri-frequency probe, a USB-C charger, and a setup guide for the probe and the echOpen On mobile application.
The My echOpen platform provides access to over 30 training sheets (flashcards), articles, videos, as well as webinars and monthly clinical case analyses (EchoClinical staff). Replays of past webinars are available.
The digital features, a cluster of services known as My echOpen, are an online platform containing training content, recorded consultations (photos, video loops), and shared consultations. My echOpen can be accessed directly from your browser.
Yes. The echOpen O1 probe has been validated in three clinical trials (Clin‑Echo 1, Clin‑Echo 2, and the Presto trial). Developed by AP‑HP, it carries CE marking (Class IIa) and complies with CE MDR and ISO 13485 standards. Probe data is securely stored on a French HDS‑certified cloud.
The echOpen O1 probe is compatible with most recent smartphones. A complete list of compatible devices is available here.
The echOpen probe is not suitable for examinations requiring Doppler, detailed musculoskeletal imaging, or superficial studies less than one centimeter deep.

Reach out to learn more
Make an appointment with our experts


















